Metal degassing is a technical intermediate step in aluminum production between the melting and casting of metals. Due to the reaction with water vapor, hydrogen is mainly dissolved in liquid aluminum. In order to prevent the formation of holes during the casting process, hydrogen is removed-the liquid metal is degassed. Usually, metal degassing is achieved by injecting an inert gas (usually argon) into the metal, which is combined with hydrogen bubbles. To make the industrial process more efficient, the size of the argon gas bubbles must be as small as possible. Currently, the most widely used method of refining bubbles is to place a mechanical rotor inside the liquid metal.
Aluminum and its alloys are susceptible to unique chemical corrosion called hydrogen-induced cracking, which is caused by the gradual diffusion of hydrogen molecules through the metal’s crystal lattice. This will produce local defects in the alloy, which greatly weakens its tensile strength and ductility, thereby increasing the risk of surface cracking. Although it is a solid, the hydrogen solubility of aluminum is negligible. However, molten aluminum is an extremely reactive substance that can actively decompose moisture to produce hydrogen.
The melt in the aluminum melt processing process may absorb hydrogen in the atmosphere and decompose the water that has accumulated or accumulated on tools such as conveying ladle or crucible furnace. If the melt absorbs excess hydrogen, it may affect the porosity of the final product and result in increased scrap. Eliminating the absorption of gas in molten aluminum is not feasible, so metallurgists work to remove hydrogen from the melt before casting.

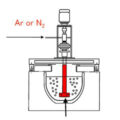
Degassing of molten aluminum is an essential process in alloy casting. It is done by one of two methods: flux or rotary degassing. The rotary degasser injects inert gas (such as argon (Ar) or nitrogen (N2)) directly into the aluminum melt. The rotation of the shaft combined with this inert gas purging results in the formation of a large number of bubbles in the melt.
The hydrogen dissolved in the molten aluminum then diffuses into these bubbles and separates from the liquid phase. Compared with flux degassing, rotary degassing unit provides a more effective and clean method for removing hydrogen from molten aluminum.