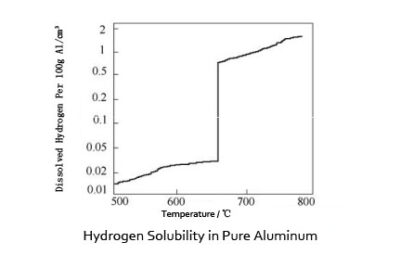
Hydrogen Influence on Aluminum is obvious. Hydrogen is the only gas that is appreciably soluble in aluminum and its alloys. Its solubility varies directly with temperature and the square root of pressure. During the cooling and solidification of molten aluminum, dissolved hydrogen in excess of the extremely low solid solubility may precipitate in molecular form, resulting in the formation of primary and/or secondary voids.
Hydrogen Sources
There are numerous sources of hydrogen in aluminum. Moisture in the atmosphere dissociates at the molten metal surface, offering a concentration of atomic hydrogen capable of diffusing into the melt. The barrier oxide of aluminum resists hydrogen solution by this mechanism, but disturbances of the melt surface that break the oxide barrier result in rapid hydrogen dissolution. Alloying elements, especially magnesium, may also affect hydrogen absorption by forming oxidation reaction products that offer reduced resistance to the diffusion of hydrogen into the melt and by altering liquid solubility.
Hydrogen Influence on Aluminum
Two types or forms of hydrogen porosity may occur in cast aluminum. Of greater importance is inter-dendritic porosity, which is encountered when hydrogen contents are sufficiently high that hydrogen rejected at the solidification front results in solution pressures above atmospheric. Secondary (micron-size) porosity occurs when dissolved hydrogen contents are low, and void formation is characteristically subcritical.
Finely distributed hydrogen porosity may not always be undesirable. Hydrogen precipitation may alter the form and distribution of shrinkage porosity in poorly fed parts or part sections. Shrinkage is generally more harmful to casting properties. In isolated cases, hydrogen may actually be intentionally introduced and controlled in specific concentrations compatible with the application requirements of the casting in order to promote superficial soundness.
Hydrogen in Solid Solution
The disposition of hydrogen in a solidified structure depends on the dissolved hydrogen level and the conditions under which solidification occurs. Because the presence of hydrogen porosity is a result of diffusion-controlled nucleation and growth, decreasing the hydrogen concentration and increasing the rate of solidification act to suppress void formation and growth. For this reason, castings made in expendable mold processes are more susceptible to hydrogen-related defects than parts produced by permanent mold or pressure die casting.
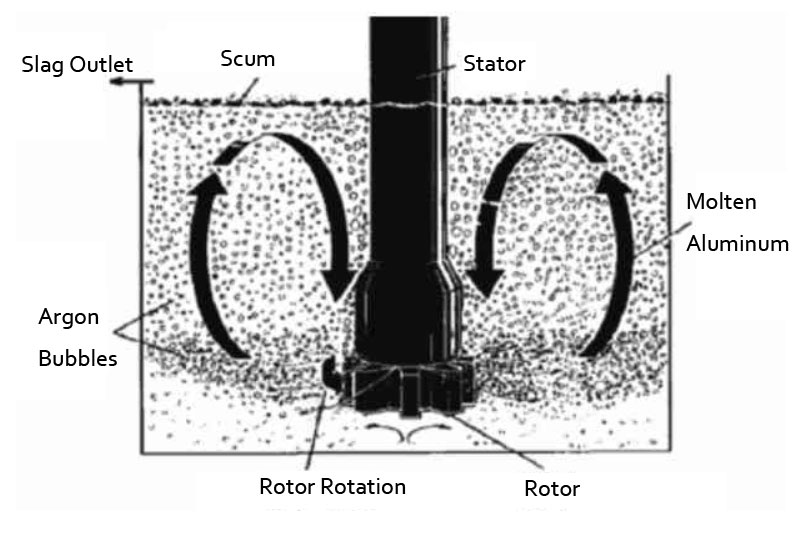
Degassing Unit for Hydrogen Removal
Dissolved hydrogen levels can be reduced by a number of methods, the most important of which is fluxing with dry, chemically pure nitrogen, argon, chlorine, and freon. Gas fluxing reduces the dissolved hydrogen content of molten aluminum by partial pressure diffusion. AdTech online degassing unit can degas by the inert gas, such as nitrogen, argon.
Inject an inert gas into the degassing box, the rotor rotates at high speed. And the large bubbles of inert gas are broken into very fine small bubbles, and they are evenly dispersed in the molten metal. By reducing the bubble diameter, the total surface area of these bubbles increases dramatically. This makes the surface of more inert bubbles come into contact with the hydrogen and impurities in the liquid metal. As the bubbles rise, the hydrogen and impurities are brought to the surface of the liquid for removal.
The flow rate of the inert gas is adjusted according to the volume of the metal liquid. The speed of the rotating rod and rotor can also be adjusted to produce bubbles of appropriate size to facilitate the diffusion of inert gas.